Results:
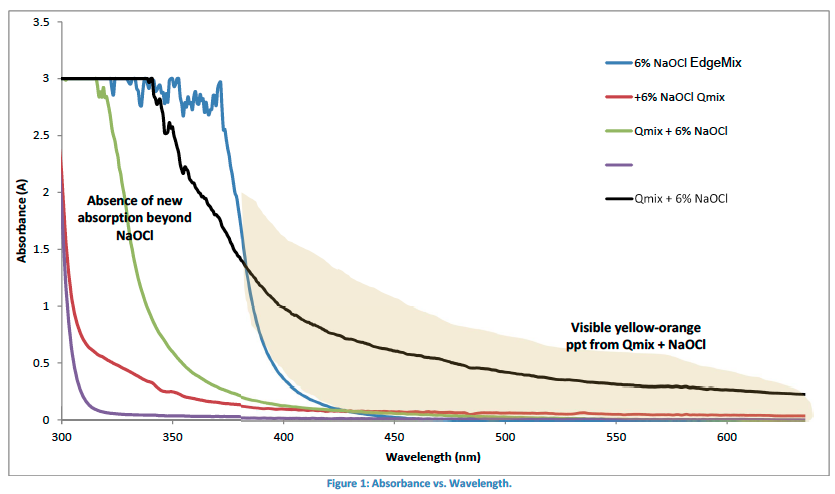
The spectra shown in Figure 1 shows that sodium hypochlorite (blue line) has an absence of absorbance past 400 nm in wavelength. The same observation is made when EdgeMix was mixed with sodium hypochlorite (green line).
Furthermore, since the absorbance spectra of EdgeMix mixed with sodium hypochlorite falls in-between the two individual spectra. This implies that no additional component species are created by their mixture. On the other hand, Qmix when mixed with sodium hypochlorite (black line) shows that it is still being absorbed even after 400nm. This shows that a color change has been noticed because it does not follow the same trend as sodium hypochlorite and EdgeMix with sodium hypochlorite. This color change also identifies that a precipitate is being formed and this means that other undesirable products are being formed when the two are being mixed together [1].
In a past study this orange/brown precipitate was characterized to be parachloroaniline (PCA) and this precipitate has been shown to be toxic [1]. Not only is this precipitate toxic, but it also can interfere with the sealing of a root filling [1]. EdgeMix was found to be compatible with sodium hypochlorite while Qmix was not compatible with sodium hypochlorite.
Read Full Study Here: Testing for Qmix and EdgeMix™ with NaOCl

